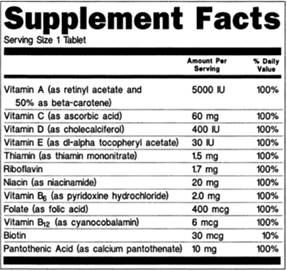
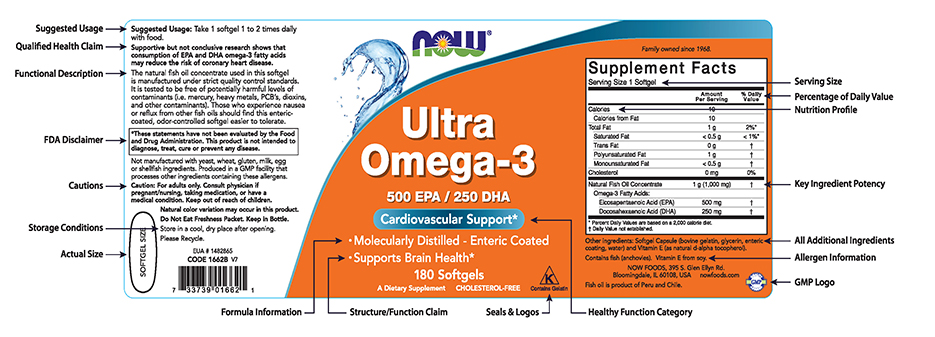
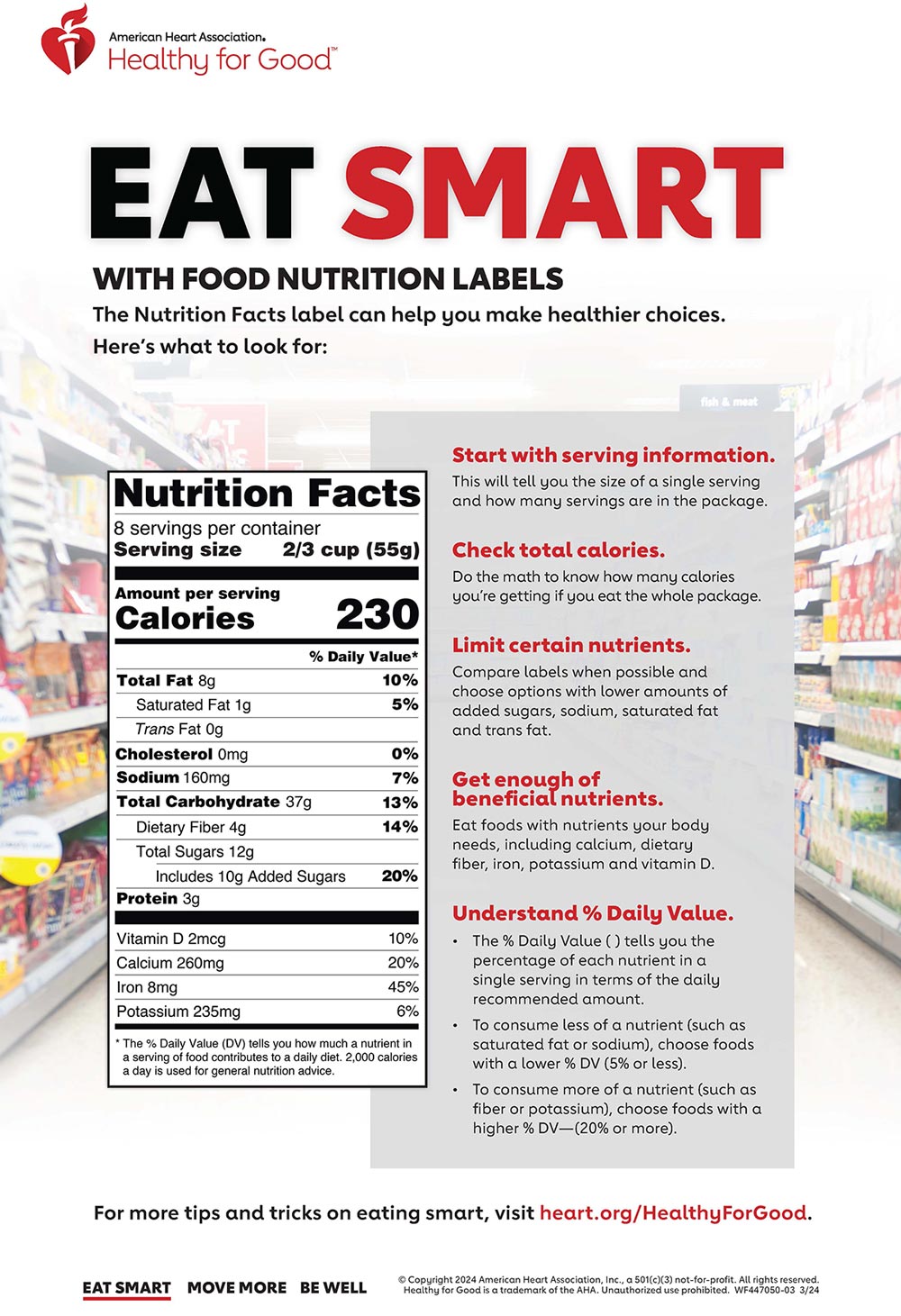
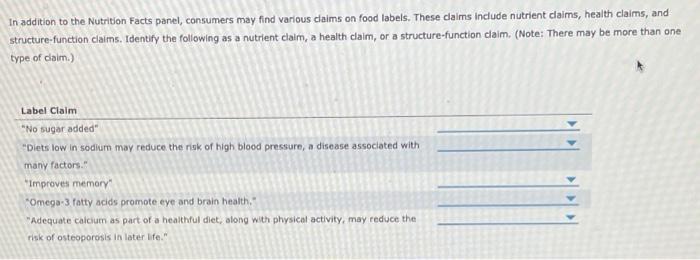
38 structure function claims on dietary supplement labels
ods.od.nih.gov › About › dshea_WordingDietary Supplement Health and Education Act of 1994 "(A) the statement claims a benefit related to a classical nutrient deficiency disease and discloses the prevalence of such disease in the United States, describes the role of a nutrient or dietary ingredient intended to affect the structure or function in humans, characterizes the documented mechanism by which a nutrient or dietary ingredient ... › food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Health claims, nutrient content claims, and structure/function claims used on food and dietary supplement labels.
› food › dietary-supplements-guidanceDietary Supplement Labeling Guide: Chapter VI. Claims | FDA What health claims can be used on dietary supplement labels? ... What must I do when making structure/function claims in my products' labeling? You must (1) have substantiation that such statement ...

Structure function claims on dietary supplement labels
en.wikipedia.org › wiki › Dietary_supplementDietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ... › structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ...
Structure function claims on dietary supplement labels. › regulatory-information › search-fdaSmall Entity Compliance Guide on Structure/Function Claims What are structure/function claims? The Dietary Supplement Health and Education Act of 1994 (DSHEA) added section 403(r)(6) ... Dietary supplement labels or labeling may, subject to the ... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements Mar 07, 2022 · The Dietary Supplement Health and Education Act of 1994 (DSHEA) established some special regulatory requirements and procedures for using structure/function claims and two related types of dietary ... › structurefunction-claimsStructure/Function Claims | FDA Mar 07, 2022 · Structure/Function Claims for dietary supplements ... appeared on the labels of conventional foods and dietary supplements as well as drugs. The Dietary Supplement Health and Education Act of 1994 ... en.wikipedia.org › wiki › Dietary_supplementDietary supplement - Wikipedia Definition. In the United States, the Dietary Supplement Health and Education Act of 1994 provides this description: "The Dietary Supplement Health and Education Act of 1994 (DSHEA) defines the term "dietary supplement" to mean a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients: a vitamin, a mineral, an herb or ...
























Post a Comment for "38 structure function claims on dietary supplement labels"